16+ Proton Neutron And Electron Calculator
1 Pounds to common mass-weight units. Mass of Proton - Measured in Kilogram - Mass of Proton is 167262 1027.

Pdf Critical Assessment Of Theoretical Calculations Of Atomic Structure And Transition Probabilities An Experimenter S View
The capacity of a battery is measured in.
. The short-lived nitrogen-16 decay emits a powerful beta ray. He suggested that a neutrally charged particle consisting of a proton and an electron bound to each other also resided in the nuclei of atoms. The mass of a proton is 16726219 10-27 kilograms.
Mass Number - Mass Number is the sum of protons and neutrons. The electric charge that is associated with a neutron is 0. Physical magic numbers and odd and even proton and neutron count.
Due to the change in the nucleus a beta particle is emitted. ASCII characters only characters found on a standard US keyboard. Therefore neutrons are neutrally charged subatomic particles.
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. The paired neutrons and protons in nuclear energy levels are filled when the number of neutrons or protons is equal to 2 820285082 or 126. The beta particle is a high-speed electron when it is a β decay and a positron when it is a β decay.
This process can be written as. Proton and a neutron If you want the answer in MeV just multiply by 931494 otherwise. This is because each neutron and each proton weigh one atomic mass unit.
Atomic mass is defined as the number of protons and neutrons in an atom where the mass of each proton and neutron is approximately 1 amu 10073 and 10087 respectively. 16 elements have 2 stable isotopes apiece. Proton Neutron and an Electron.
Must contain at least 4 different symbols. The most common scales are the Celsius scale with the unit symbol. The presence of neutral particles in the nuclei of atoms was also suggested by Ernest Rutherford in the year 1920.
An X-ray or much less commonly X-radiation is a penetrating form of high-energy electromagnetic radiationMost X-rays have a wavelength ranging from 10 picometers to 10 nanometers corresponding to frequencies in the range 30 petahertz to 30 exahertz 3 10 16 Hz to 3 10 19 Hz and energies in the range 145 eV to 124 keVX-ray wavelengths are shorter. The mean number of stable isotopes for elements which have at least one stable isotope is 25180 31375. In this model all the nuclear particles are paired one-to-one neutron with a neutron and proton with a proton.
Neutron interactions with most types of matter in this manner usually produce radioactive nuclei. 6 to 30 characters long. Option iv is the answer.
Protons carry a positive charge and are found in the nucleus of an atom. 1 lbs 0453592 kilograms kg 1 lbs 453592 grams g 1 lbs 453592 milligrams mg 1 lbs 1 pounds lbs 1 lbs 16 ounces oz 1 lbs 226796 carats ct 1 lbs 69999953702918 grains gr 1 lbs 0071428548932411 stones st 1 lbs 34999971450137 scruples 1 lbs 0035714256581772 quarters UK 14UK. Atomic Number - Atomic Number is the number of protons present inside the nucleus of an atom of an element.
Iv The two electrons present in the 2s orbital have spin quantum numbers. The mass of an electron corresponds to an energy of around 81871014 joules or about 05110 MeV. Mass Defect - Measured in Kilogram - Mass Defect is the difference between the actual atomic mass and the predicted mass.
Formula of converting mega electron volts to electron volts. The mass of a neutron is roughly equal to 1008 atomic mass units. These are the magic numbers that show the most stable nuclei.
These last 26 are thus called monoisotopic elements. Interactive periodic table showing names electrons and oxidation states. It comprises 4 neutrons and 1 proton.
26 elements have 1 single stable isotope. 0038 18_8O 8. Visualize trends 3D orbitals isotopes and mix compounds.
Therefore an online average atomic mass calculator can find the atomic mass of any element by adding the number of neutrons and protons. The mass of an electron is not taken into account when determining an atoms mass number. Similarly if a neutron is converted to a proton it is known as β decay.
ResearchGate is a network dedicated to science and research. It decays through triple neutron emission into hydrogen-3. It has been incorporated in the laboratory by bombarding tritium with fast-moving tritium nuclei.
When converted into kilograms the mass of the neutron can be approximated to 167410-27 kg. It comprises 6 neutrons and 1 proton. It has a half-life of 290 yoctoseconds.
He coined the term neutron to refer to these neutrally charged particles. Since the vast majority of an atoms mass is made up of its protons and neutrons subtracting the number of protons ie. A neutron in the nucleus splits into a proton and an electron on the emission of a beta particle.
Ii An electron in the 2s orbital has the same energy as an electron in the. The atomic number from the atomic mass will give you the calculated number of neutrons in the atom. Binding Energy formula is defined as the amount of energy required to separate a particle from a system of particles or to disperse all the particles of the system is calculated using Binding Energy Atomic Number Mass-pMass Number-Atomic Number Mass-n-Mass of atomc2To calculate Binding Energy you need Atomic Number Z Mass Number A.
Temperature is measured with a thermometer. This calculator uses a formula when performing the conversions. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition.
Connect collaborate and discover scientific publications jobs and conferences. Subtract the atomic number from the atomic mass. Beta particles are used to.
Or case of a mercury cell is the positive terminal - Have a look at your watch or calculator battery to verify. Hence it is the electron that is emitted by the nucleus at a rapid pace. Fluorine 19_9F 9.
Iii Zeff for an electron in 1s orbital is the same as Zeff for an electron in a 2s. In comparison to a proton or a neutron an electron is considered nearly massless. Mass of the nucleus of 816O Mass of 8 protons Mass of 8 neutrons 8100757810089316132amu Mass defect 16132.
Beta particles have a higher penetration power when compared to alpha particles and. Hydrogen-5 is a highly unstable isotope of hydrogen. It weighs around 91091031 kilogrammes or 5486104 daltons.
If a proton is converted to a neutron it is known as β decay. The proton and the neutron are approximately the same mass neutron very slightly heavier. The abundant oxygen-16 nucleus for example undergoes neutron activation rapidly decays by a proton emission forming nitrogen-16 which decays to oxygen-16.
The electrons within the atom are so tiny compared to the protons and. Atoms contain three types of subatomic particles called protons neutrons and electrons. Ms but of opposite sign.
The numbers after the decimal point represent the usually very small mass of the electrons in. Neutron neutron degeneracy neutron star Newtons first law Newtons second law Newtons second law for rotation Newtons third law Nortons theorem nuclear binding energy Ohms law orbit circular orbit concepts orbit velocity orbital angular momentum orbital quantum number organ of Corti oscillator simple harmonic.
Atomic Mass Calculator Online Solver With Free Steps

Metal Oxo Cluster Formation Using Ammonium And Sulfate To Differentiate Miv Th U Ce Chemistries Inorganic Chemistry

Formation Of Gaseous Peptide Ions From Electrospray Droplets Competition Between The Ion Evaporation Mechanism And Charged Residue Mechanism Analytical Chemistry
![]()
Best 10 Apps For The Periodic Table Of Elements Last Updated December 9 2022

Element Z Has Two Isotopes Their Mass Numbers Are 57 And 59 The Percentage Abundance Of Each Isotope Is 75 Of 57z And 25 Of 59z What Is The Relative Atomic Mass

How Does The Higgs Boson Give Mass To Subatomic Particles Like Protons And Antimatter Particles Like Positrons And Anti Protons Quora
Element X Has Two Isotopes Of 16 And 18 Its Relative Atomic Mass Is 16 4 What Is The Percentage Abundance Of Each Isotope Quora
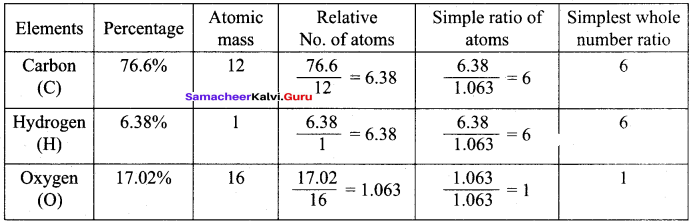
Samacheer Kalvi 11th Chemistry Solutions Chapter 1 Basic Concepts Of Chemistry And Chemical Calculations Samacheer Guru
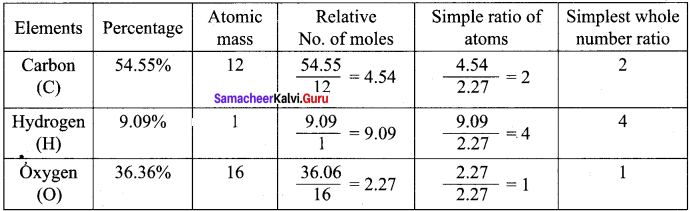
Samacheer Kalvi 11th Chemistry Solutions Chapter 1 Basic Concepts Of Chemistry And Chemical Calculations Samacheer Guru

Atomic Mass Calculator Calculate Neutrons Protons Electrons

Mcat All Set 1 Physics Bio Ch1 6 Flashcards Quizlet

Atomic Mass Calculator Calculate Neutrons Protons Electrons

Oxford English Picture Dictionary Enlish Arabic Ismail Rao Page 1 317 Flip Pdf Online Pubhtml5

Sulfolane Induced Supercharging Of Electrosprayed Salt Clusters An Experimental Computational Perspective Journal Of The American Society For Mass Spectrometry

Calculating The Protons Neutrons And Electrons For An Atom Youtube

Pdf Expanding Nuclear Physics Horizons With The Gamma Factory Dmitry Budker Academia Edu

Atomic Charge Calculator Calculator Academy